Cloward H2O, an expert in aquatic design, has been diving into the important topic of breakpoint chlorination and has shared insights from project manager Brad Clawson.
Chlorine is a code-required element in all public recreational water facilities. Often, a residual level of 1 – 2 ppm is required to be maintained at all times.
"When chlorine is added to water for normal residual chlorination, it combines (reacts) with ammonia and organic contaminants found in pool water (sweat, urine, skin cells, and other organic matter), forming 'combined chlorine' compounds commonly called 'chloramines'," explains Clawson.
The terms 'combined chlorine' and 'chloramines' are often used interchangeably and refer generically to a family of chlorinated compounds.
"It is the build-up of combined chlorine that swimmers most often notice in a pool, complaining of a 'chlorine smell' overhanging the pool surface and eye and nose irritation. Interestingly, a common misconception is that this “chlorine smell” is due to large amounts of unnecessary chlorine in the water when it is actually due to an insufficient amount of chlorine in the water to properly oxidize the contaminants."
In addition to residual chlorination, chlorine is used to 'shock' or super-chlorinate pool water to break down contaminating organic compounds, kill algae and other microorganisms, destroy impurities and dissolved waste products, and break apart the chemical bonds forming combined chlorine.
The 'breakpoint' is the chlorine concentration that breaks these chemical bonds. Breakpoint chlorination removes chloramines and other reductants that raise water chlorine demand.
The breakpoint
A ratio of 7.6:1 free chlorine to combined chlorine molecules is needed to achieve breakpoint. Molecules of mixed chlorine are broken down and destroyed at or above this ratio.
In order to achieve breakpoint, a ratio of 7.6:1 free chlorine to combined chlorine molecules is required. At or above this ratio, combined chlorine molecules are broken down and destroyed.
"Reaching the breakpoint is an all-or-nothing reaction. Not adding enough chlorine to reach breakpoint will result in more combined chlorine and lower free chlorine residual. When completed, breakpoint chlorination destroys the chemical bonds with ammonia, leaving free chlorine, nitrogen, water, and inorganic chloride (salt)."
Several chemical reactions take place before breakpoint is achieved:
- Free Chlorine (HOCl) reacts with Ammonia (NH3) to form Monochloramines (NH2Cl).
- HOCl also reacts with NH2Cl to form Dichloramines (NHCL2) and further with NHCL2 to form Trichloramines (NHCL3) or nitrogen trichloride (NCl3) and Nitrates (NO3).
- NCl3 forms when the HOCl to nitrogen molecular weight ratio is greater than 12:1. When this happens, oily, insoluble colloidal particles appear and cloud the water. They then migrate toward the water's surface and may be released into the air.
When the chloramine level gets to 0.2 ppm or higher, the pool water should be shocked or super-chlorinated to the point of breaking. Some types of chlorine, like Trichlor or Dichlor, can't be used to hit the breakpoint because they are stabilised. Using these items for breakpoint chlorination would add too much cyanuric acid to the water solution concurrently stopping the full reactions from happening.
"Before attempting breakpoint chlorination, make sure that the water is chemically balanced and that the pH is between 7.2 and 7.4 to maximize the percentage of hypochlorous acid formed," explains Clawson. "Shock treating a pool with unbalanced water, particularly with a high (basic) pH or high total alkalinity, will result in the formation of a white carbonate precipitate that will cloud the water.
"However, some operators prefer to raise the pH when using acidic chlorine products, like elemental gas chlorine, for super-chlorination since more offensive forms of chloramines develop rapidly at a very low pH."
Making the right calculations
To calculate the breakpoint concentration necessary to super-chlorinate, a DPD (N, N-Diethyl-P-Phenelynediamine) or FAS (Ferrous Ammonium Sulfate) test kit can be used to find both the free and total available chlorine levels.
"Subtract the Free Available Chlorine (FAC) from the Total Available Chlorine (TAC) to find the Combined Available Chlorine (CAC) level. Multiply the CAC by a factor of 10, although only 7.6 is actually needed, to find the dose of chlorine you must introduce into the pool in order to reach the breakpoint.
"Ten is used as a factor because most pool operators are not sure of the precise amount of water in their pools, or of the exact percentage of available chlorine in the chlorine compound being used. Using ten as a factor errs on the side of caution and helps ensure that enough chlorine is left over after breakpoint has been achieved to satisfy the chlorine demand and leave an adequate residual."
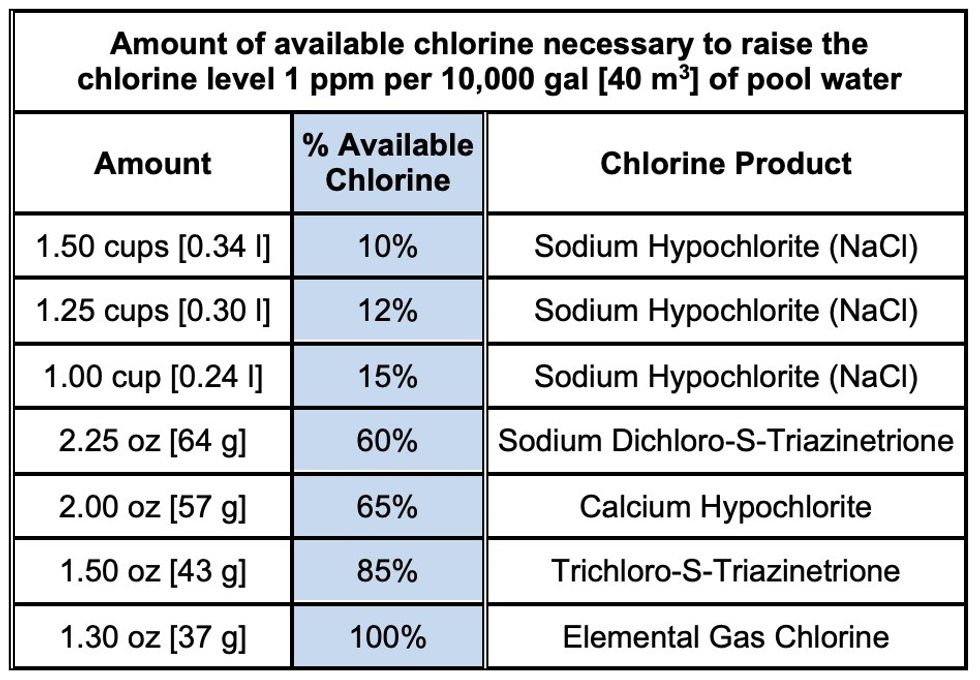
Operators should find out how many gallons of pool water need to be cleaned and what percentage of chlorine is available in the product that will be used to totally chlorine the pool. They can use a standard chart or a chart from the chlorine maker to figure out how much chlorine they need by weight.
Best times to super-chlorinate
Clawson provides an example:
- 83,000-gal pool
- 1.2 ppm Free Chlorine and 1.8 ppm Total Chlorine Measured in the pool water
- 10% NaCl Solution to be used => 1.50 cups/10,000 gal/1 ppm dose (from chart)
- Subtract Free Chlorine from Total Chlorine: 1.8 ppm – 1.2 ppm = 0.6 ppm Combined Chlorine
- Multiply Combined Chlorine by 10: 0.6 ppm * 10 = 6.0 ppm Chlorine required to reach Breakpoint
- Thus: (8.34 lbs/gal of water)*(6 ppm)*(1.5 cups) = 75.0 cups ÷ 16 cups/gal = 4.7 gallons of 10% chlorine solution required.
When chlorine concentrations are high, swimmers may not be allowed to use the pool according to certain health department restrictions.
"It is best to super-chlorinate in the evening or during hours the pool is not in operation to avoid respiratory irritation to users from off-gassing during the super-chlorination process and to allow chlorine levels to drop back to normal levels," says Clawson.
The pH, pool water temperature, the ratio of free accessible chlorine to combined chlorine, the content of ammonia/nitrogen and organic nitrogen molecules that demand chlorine, and other factors all affect how quickly the breakpoint is achieved. The substantial quantities of chlorine added to the water will be consumed if the chemical reaction occurs and the breakpoint is achieved. The mixed chlorines will be removed, and free chlorine will be restored to typical operating levels.
Cloward H2O also recently shared insights into the issue of salt versus chlorine, a commonly asked question regarding pool water disinfection.
Charlotte Coates is blooloop's editor. She is from Brighton, UK and previously worked as a librarian. She has a strong interest in arts, culture and information and graduated from the University of Sussex with a degree in English Literature. Charlotte can usually be found either with her head in a book or planning her next travel adventure.